Need Leads to Innovation
Back in the year 2000, Craig Hershoff, the man developed the robot, was diagnosed with Fuch’s dystrophy. For this, the man almost lost his eyesight. However, after three corneal transplants over the next ten years, the man was finally able to keep his ability to see, although he needed high-powered lenses. Now, after the passing of his wife, Hershoff had pretty difficulty in inserting the lenses into his eyes as he was going through a period of anxiety. As a result, his hands shook and he could not place the contact lenses in his eyes properly. And this was an important point of life for him. “What happens in a few years if I actually have a tremor and I can’t get these lenses in? I need them to see and I don’t have anyone to help put them in for me,” Craig told in an interview with CNN. So, to help himself insert and remove his eye-lenses, Hershoff developed the “Cliara” robot. Now, although the name “Cliara” is pretty cool in itself, it is actually an acronym for “Contact Lens Insertion and Removal Apparatus”. “What is so unique and special about this device is that there is a camera attached so you can see where the lens is going and exactly how it’s being placed. Any type of anxiety or nervousness is gone because you are controlling the device and it’s extremely gentle and safe.”, he continued.
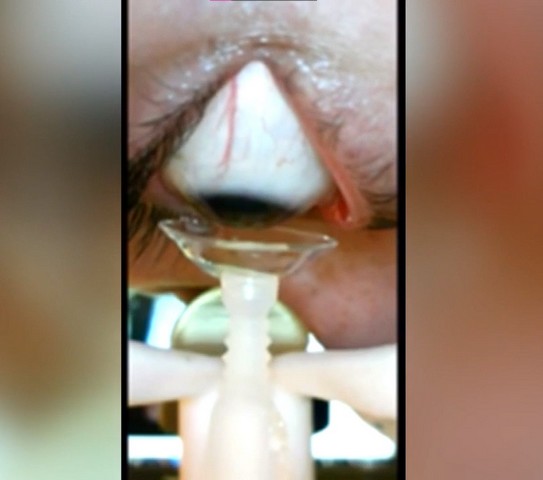
How Does It Work?
Now, according to its maker, the robot uses special suction cups to measure the amount of pressure needed to insert or remove the lenses. Moreover, the user will be able to control it themselves and monitor the eye into which they inserting the lens in real-time. This will help the user to “track the motion of the contact lens at all times”.
“When they are ready, the user commands the device to go up to the eye and very sensitive force sensors detect contact and stop the motion of the device as the contact lens is inserted. After insertion, the device retracts downward.”, Hershoff further added. Now, as per the maker, the Cliara robot is currently in its testing stage and is going through many clinical trials. And Hershoff plans to get the device ready for the consumer market by the first half of next year. However, he needs the FDA (Food and Drug Administration) approval prior to the start of mass-production.